1/6/22 (Author’s note, contact)
Introduction

“Agree with @CarlosdelRio7 we should not use on demand TAF/FTC for PrEP until we have data since rectal tissue concentration of TVF-DP seem lower than with TDF/FTC according to #CourtneyFlechtner at #CROI2019”
Jean-Michel Molina, via Twitter, 3/9/19
…And so it is that the question of efficacy of Descovy™️ for HIV prevention with nondaily oral pre-exposure prophylaxis (PrEP) dosing has come to be asked over and over again. That’s been the case at least since initial DISCOVER trial results were announced in Spring 2019, followed by limited F-TAF daily-only PrEP approval by the US Food and Drug Administration (FDA) four months later.
“The U.S. Food and Drug Administration today approved Descovy (emtricitabine 200 mg and tenofovir alafenamide 25 mg) in at-risk adults and adolescents weighing at least 35kg for HIV-1 pre-exposure prophylaxis (PrEP) to reduce the risk of HIV-1 infection from sex, excluding those who have receptive vaginal sex. Descovy is not indicated in individuals at risk of HIV-1 infection from receptive vaginal sex because the effectiveness in this population has not been evaluated.”
FDA news release dated 10/3/2019
[CAVEAT: This F-TAF 2-1-1 oral PrEP conversation excludes many who would benefit from more flexible PrEP offerings. In fact, the bolded emphasis added above is due to the fact that cis women and many Trans women, Trans men, other folks assigned female at birth, people who inject drugs, people of color, and youth have already largely been left out of this early F-TAF oral PrEP efficacy research. This exclusion explains in part why the approval was not a fuller one like F-TDF’s oral PrEP approval. A related critical conversation is ongoing. Accordingly, Gilead – the company that makes Descovy™️ – has committed to correcting this erasure and started some such inclusion. However, time will tell whether or not the question of F-TAF 211 oral PrEP efficacy addressed herein will apply meaningfully to ANY of these priority groups vulnerable to HIV acquisition.]
Definitions

The specific drug in question is Gilead’s slightly chemically updated Descovy (Tenofovir-alafenamide/Emtricitabine), a combo HIV antiretroviral treatment component pill launched to replace the 2017-patent-expiring Truvada [Tenofovir-disoproxil fumerate (TDF)/Emtricitabine, or F-TDF]. TAF is just a prodrug or derivative of TDF. (Some argue that it’s merely an evergreened derivative at that, thus the newly-patented-product launch.)

(The current US HIV treatment guidelines draw the distinction between TDF and TAF as such, “TAF and TDF are two forms of TFV [Tenofovir] approved by the FDA. TAF has fewer bone and kidney toxicities than TDF, whereas TDF is associated with lower lipid levels. Safety, cost, and access are among the factors to consider when choosing between these drugs.” Liver-, bone-, and kidney health – factors identified above – are important considerations for people taking therapeutic ARVs over long periods, especially as those people age.)
In addition to its patent-related F-TDF-to-F-TAF sales switch strategy for PrEP consumers, Gilead has also marketed Descovy to care providers as a preferable option for PrEP patients on ARVs as preventatives for long periods as they age. (This switch to the newly-patented prodrug means for Descovy a period of time with no price-lowering competition from generic versions.) Thus, the distinction mentioned above in current US HIV treatment guidelines helps explain why some PrEP users (and their care providers) opt for one (or the other) drug for oral Tenofovir-based PrEP.
From a consumer perspective though, I suspect that deciding whether to opt for or switch from/to daily- vs 2-1-1 PrEP generally overlaps with rationales (‘economical, safe, effective, simple, and flexible’) asserted among some PrEP users who have resorted to the Ts and Ss PrEP approach. (Ts and Ss PrEPping is another 4-pill-based, nondaily oral PrEP dosing strategy adapted among cis MSM, involving “weekly-base” dosing or more aptly “routine-plus-event-based” dosing on Tuesdays, Thursdays, Saturdays, and Sundays regularly).
The specific nondaily PrEP dosing approach in question herein is the popularly-coined “2-1-1” dosing strategy (also referred to sometimes as event-based/event-driven/intermittent dosing, on-demand dosing, pericoital dosing, or IPERGAY dosing). This approach using oral F-TDF has been demonstrated as highly effective in multiple trials among cisgendered men in the receptive position during penetrative, condomless anal sex (IPERGAY, IPERGAY OLE, AmPrEP, the San Francisco study, PREVENIR, etc). It starts with two pills 2-24hrs before sex, a 3rd pill 24hrs after the 1st two pills, and a 4th pill 24hrs after the 3rd pill (see diagrams below).


As the diagrams above also show, the approach allows for flexibility of switching back and forth with daily- and 2-1-1 dosing if needed. This is true as long as there are the post-sex doses 24hrs then 48hrs after last sexual contact, and even with a single pre-sex loading dose instead of two if there was any drug dosing within the previous 7 days. (Though the World Health Organization recently warned that, through experience, the French had to simplify that restart to the usual two pills, regardless of whether or not the gap is less than 7 days or more than 7 days.)
TL:DR
Now, it should be pointed out that the idea of less-than-daily dosing was hinted at indirectly in the FDA application for Descovy™️’s PrEP approval back in 2019. The rationale for the regulator’s rejection of the idea can be surmised from the following excerpt relevant to cis women:
“As part of its rationale for utilizing systemic drug exposure data to support a PrEP indication in cisgender women, the Applicant also states that TAF can quickly (within 2‐3 hours following a single dose) achieve TFV‐DP concentrations of 40 fmol per million cells in PBMCs of both men and women, a clinical threshold demonstrating a level of adherence reportedly associated with a greater than 90% reduction in HIV acquisition (EC90) in prior PrEP trials of Truvada {Anderson et al. 2012a; Anderson et al. 2012b}. However, while this target concentration may be valid for MSM receiving F/TDF, it may not apply to F/TAF due to the differences between TDF and TAF in the correlation between PBMCs and mucosal tissue concentrations for TFV‐DP.”
FDA briefing document, 8/7/2019, pg 26
In other words, as Molina pointed out earlier, “rectal tissue concentration of TVF-DP [with TAF/FTC] seem lower than with TDF/FTC.” Indeed, with IPERGAY™️ trial lead investigator Jean-Michel Molina’s response to the query again via Twitter in early 2020 (screenshotted below), the question of F-TAF 211 PrEP efficacy had again percolated up to one of the most authoritative voices on the topic of 211 PrEP efficacy.

Molina’s response, “…[T]here is not [data to support event-based- or timed F-TAF PrEP] and I would not recommend event-driven TAF/FTC until we have good data” just about sums up any TL:DR one could offer with the very same reason why F-TAF 2-1-1 PrEP approval could not be advanced by the FDA.
And yet, the question has popped up more and more frequently in the U.S.-based PrEP Facts Facebook group and elsewhere online as Descovy™️ (F-TAF) has taken more and more market share from Truvada™️ (F-TDF). In other words, there is definitely demand for this (and almost certainly execution already too). Thus, a more nuanced but still reasonable TL:DR to such a question in the category of challenging-because-there’s-no-authoritative-data might read as such:
‘We just can’t say for sure now whether or not the 2-1-1 oral PrEP protocol works with F-TAF to prevent HIV acquisition since A.) F-TAF oral PrEP is only known to be effective/approved/recommended as a daily regimen for people assigned male at birth (AMAB) and B.) there’s no such 2-1-1 F-TAF oral PrEP randomized control trial data yet in humans. Nevertheless, since there are some encouraging bits of animal challenge trial data and human pharmacology data, it’s obviously your-body-your-choice for those people who will do what they will do regardless of the admonitions like that from the esteemed authority quoted above. So, in such cases, it’s best that they try to get balanced, unbiased, comprehensive evidence-based information and discuss it with their care providers for making health and medical decisions that fit their own lives.’
Roadmap
Now, you, dear reader, might be wondering why I am still interested in this topic (or why you should be) beyond the TL:DRs articulated above. Well, if you’re at all like me, then you can’t help but see the demand-but-no-data dilemma. You might also, like me, be excited about new biotechnologies (TAF) and new related approaches TAF-based PrEP, 2-1-1 PrEP), especially when they’re begun or at least based in the community as opposed to the academy. You might even have an eye to the harm reductionist care provider’s approach of shared decision-making by telling-what-you-know and applying the info collaboratively to a patient’s needs. Or, you might just like deep dives that give greater background, context, and detail on such topics. So, with that, feel free, if you’d like, to review with me some of the lead-in (the terms and history), the logic (inferential data), and the limitations (data/guidance gaps) that cause an intrepid few to take a more optimistic (some say permissive) view of people considering experimenting on their own with F-TAF 2-1-1 PrEP while causing many others to dismiss this whole idea as speculative at best. For, in reviewing such information, we might cover some of the balanced, unbiased, comprehensive evidence-based tangential information necessary in order to address the dilemma of such demand-but-no-data drug dosing decisions.
THE LEAD-IN (BACKGROUND)
In order to understand how we got to the point where there were two PrEP pills, multiple dosing strategies, and a desire/need to use one of those pills like the other but without corresponding evidence of efficacy, we’ve got to go back to about 1999. That’s when TAF was just a TDF-offshoot lab compound codenamed GS-7340, sloshing around in vitro. (A quick-n-dirty enumeration on PrEP’s overall history can be found here.) It would take two years, until 2001, for first published papers introducing TAF to the world, while TDF was already making its market debut for HIV treatment. This was soon followed by the start of TAF human testing in 2002 for HIV treatment and the 1st TDF testing as a daily prevention pill in people as well in 2003.
Meanwhile, 2004 brought a curious TAF research pause just as the FDA approved a combined F-TDF pill for HIV treatment. The F-TDF pill approval dove-tailed well, two years later, with animal research showing better preventative power in the combined F-TDF approach rather than either ARV alone and led to a research shift in the form of a combined/comparative approach in the subsequent daily oral TDF-based PrEP trial launched in 2008.
(It doesn’t go without notice also that 2005 saw a TAF-focused expression of hope acknowledging that “better intracellular distribution of the oral prodrug [TAF] … [leads to] extremely potent in vitro activity and selective targeting to lymphoreticular tissues and PBMCs in vitro and in vivo … [and perhaps] effect[s] on latently infected cells …[and] latent cellular reservoirs …leading to the conclusion that it may … be possible to eradicate [HIV].” In other words, some scientists were initially so impressed with TAF’s targeted cellular penetration and potency, that they thought it might have a role to play in reaching the HIV viral reservoir in its many nooks and crannies, thereby helping totally eradicate HIV from the body.)

A series of news articles in 2003, 2005, 2006, 2007, and 2008 warned of intrepid street pharmacists, permissive care providers, and risk-oriented (some might say desperate) healthcare consumers experimenting with nondaily oral TDF-based prophylaxis at this same time that TDF was gaining a bit of a reputation for taxing the kidneys of people taking it daily for HIV treatment (and it was pointed out later, taxing their bones too). This burgeoning infamy harkened back in the psyche of the AIDS generation to the toxicity and related controversy surrounding the initial dosing of the ubiquitous 1st HIV treatment ARV known as Zidovudine/ZDV or Azidothymidine/AZT (as well as other similarly troublesome ARVs no longer marketed or commonly recommended to certain groups). That perception – fed by class action lawsuits targeting longterm safety of oral TDF-based HIV treatment regimens – later informed consumer interest in TAF as a safer oral PrEP alternative to TDF. (Though it’s worth pointing out that TDF-based oral PrEP was found to be comparatively safe to placebo as far as serious adverse events in a meta-analyses of almost 16,000 oral TDF-based PrEP research participants spanning 4mos to 4yrs from the earliest West African- and US Safety studies all the way through IPREX, PARTNERS PrEP, and the Bangkok Tenofovir Study right upto IPERGAY and PROUD.)
Subsequent to all the media attention to “the streets,” (documented from a cultural anthropological or communications perspective as a consumer demand in all the “MTV,” “Taking a T,” “the 3 Vs,” “party packs,” and “disco dosing” jargon captured in the media throughout the mid 2000s), 2-1-1 PrEP dosing was finally hitting the research/development pipeline agenda in earnest after a late 2009 researcher confab acknowledging, perhaps euphemistically, that “[T]here is considerable interest in intermittent use of PrEP (iPrEP)…People at risk of contracting HIV cannot afford to let biomedical and behavioral PrEP research be delayed.” Next, in 2010 and 2011, largescale, definitive evidence of daily, oral F-TDF’s HIV prevention power finally emerged from the R-&-D pipeline (after a series of fits-and-starts obstacles).
This F-TDF PrEP proof news was just in time, conveniently, for TAF’s 2011 R-&-D relaunch (and combining with emtricitabine). And, as the famous French IPERGAY trial launched in early 2012 with F-TDF among cis-MSM (and one trans woman), F-TDF daily PrEP was greenlighted by the US FDA. The IPERGAY trial ended surprisingly early (and successfully) about two-and-a-half years later in late 2014. Then, its Open Label Extension about two-and-a-half years hence in 2016 (around the same time as Descovy’s approval for HIV treatment and its market positioning as a more kidney-/bone favorable ARV combo compared to F-TDF and launch of DISCOVER, the only completed F-TAF PrEP trial to date, whose early (and successful) unblinding at the beginning of 2019 preceeded the limited 2019 US FDA Descovy PrEP approval excerpted early herein. (More on its results below.)
In summary – from TAF as a TDF prodrug, to TAF’s R&D pipeline entry, to TAF’s TDF coattail-riding on ARV combo-partner FTC, to related HIV treatment approvals and subsequent PrEP research positionings, to safety concerns (some primarily residual from earlier ARVs) and related consumer-driven dosing innovation demands, to treatment- and prevention trial successes followed soon by FDA approvals – this is how we got to the question of whether or not F-TAF can be used like F-TDF for this particular nondaily dosing approach.
THE LOGIC (INFERENTIAL DATA)
For many people prescribed oral F-TAF for PrEP, the 2-1-1 dosing regimen question is one of convenience, “Can I do that with what I’m already on if the drugs are similar?”
The New ‘New Thing’
Some users may just get excited about being on the vanguard of technology (like the so-called “early adopters” from the Diffusion of Innovations theory discussed by IPREX lead investigator Dr. Robert Grant). In other words, these “early adopters” may prize F-TAF as a status symbol.
Great Things … Small Packages
The tiny pill form of F-TAF may create a sense of cuteness. That cuteness often evokes the same sense of joy, whimsy, ornate decoration, and delight expounded upon recently by NPR’s This American Life yarnster Bim Adewunmi on a episode called “#687: Small Things Considered,” all about our glee in such delicate things. The tiny cuteness of F-TAF may also evoke a sense of discreteness, discreetness, and discretion that dovetails well with the taking-control-in-tiny-increments nature of the 2-1-1 PrEP dosing approach. (There could even be an overlapping “echo” of intimacy from the sex itself to the safety method of 2-1-1 via the small pill size and this simple wraparound dosing method.)
Packing A Potent (Pharmacological) Punch
Regardless, what’s clear is that F-TAF packs a powerful punch in a petit preventative/therapeutic pill package. Specifically, the comparison of Tenofovir inclusion is roughly an order of magnitude (10-fold) smaller in Descovy (25mg of TAF) vs Truvada (300mg of TDF). TAF does this by delaying its metabolism or transformation until it gets inside circulating- and stationary immune cells, the very groups that HIV targets primarily for copy-hijacking and cell-to-cell hopping systematic distribution and establishment.

In addition to many fewer mg TAF than TDF needed to penetrate and protect immune cells, TAF seems to do that cellular penetrating and protecting molecularly faster (1-2hrs vs. 3-4days), at higher levels (404 fmol vs. 49 fmol) and for longer (16 days vs 10 days than TDF).

That potency is why oral F-TAF pills are relatively so small and why some are speculating (optimistically even in certain corners) about F-TAF 2-1-1 dosing. Indeed, the biochemical angle (outlined in key documents and slide presentations for the FDA’s F-TAF oral PrEP approval proceedings) forms the greater heft of logic underpinning the speculation that oral F-TAF might be a better fit with those for whom daily adherence is impossible or not preferable otherwise.

(As a ‘potency/packaging pun intended’ aside, it’s rather curious that Descovy is marketed in single-serving “Day Tracker” blister packs that, ironically, promise longer home shelf-life and ease of portability suitable to 2-1-1 dosing than a 30-pills-for-daily-use bottle, as has been the common commercial container for its counterpart Truvada.)
The Add-On Emtricitabine
Emtricitabine’s rapid cellular onset in anal tissues also plays an important (perhaps independent) but often overlooked role in 2-1-1 PrEP dosing. FTC does this by offering initial local protection therein, early after dosing. AIDSMAP’s Gus Cairns characterized IPERGAY investigator Jean-Michel Molina this way on the matter shortly after confirmatory pharmacological data was published: “…in previous studies, 96% protection from HIV was reached after just three doses of Truvada PrEP and 99% after five doses. Adequate concentrations in blood of both of the drugs in Truvada, tenofovir and emtricitabine, were reached as soon as two hours after taking a pill but that while similar levels of emtricitabine were reached in rectal secretions in the same timespan, tenofovir did not become detectable in rectal secretions until 24 hours after the first dose. This may mean, Molina commented, that emtricitabine offers the sole protection against HIV very early on after a first dose [emphasis added].”
(The FTC finding above dovetails well with more recent complementary pharmacological data. That more-recent, relevant data broke ground on longstanding questions of PrEP drug concentrations in local penile tissues as well as questions of the relative contributions of FTC vs. TDF in local vs systemic protection. As such, these newer data echo the same Molina FTC-local PrEP “sole protection” observation. They also suggest that the protective contribution of TDF or TAF to the two-drug oral PrEP combo may be more systematic than previously supposed, unlike FTC [or its own sister-compound, lamivudine/3TC as the case may be]. Many avid readers will remember this latter local-vs-systemic question as a driving force justifying the launch and execution of the controversial DISCOVER trial, whose results – one might also now reasonably conclude in light of this newer data – also support and advance the supposition of systemic TDF/TAF PrEP protection put forth in the newer data.)
Evidence from Animals?
A few among the bold have taken to citing the limited-but-helpful role that relevant animal data can play where gaps exist otherwise; in this case, F-TAF-based event-driven PrEP efficacy data among macaques. The study actually involved a series of comparisons among variously-timed F-TAF PrEP- and PEP regimes, with- and without the addition of bictegravir (BIC). The relevant regime among them – intermittent F-TAF administered 2h prior to viral exposure and then again 24h post exposure – demonstrated significant protection in the rectal exposure model. The findings also referenced and echoed earlier F-TDF intermittent PrEP animal data that paved the way for IPREX and IPERGAY as well as other animal data that led to DISCOVER, which might explain in part how it started popping up in related discussions online.

The Proof is in … Well, the Proof?
Ever since Gilead first introduced the world to DISCOVER trial F-TAF oral PrEP data in early 2019 with “Compared to TDF, tenofovir alafenamide (TAF) has higher intracellular tenofovir (TFV)-DP levels, lower plasma TFV levels, and improved renal and bone safety when used for HIV treatment,” it’s been putting its resources behind positioning Descovy as the new-and-improved replacement for Truvada for PrEP (much like its Apple-self-cannibalizing strategy for its updated treatment products). The stunning DISCOVER trial results sure seemed to capture the imagination too.
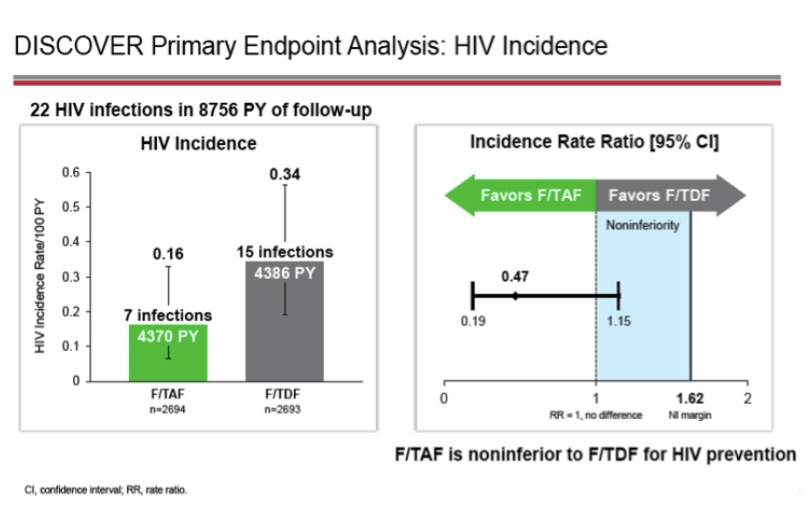
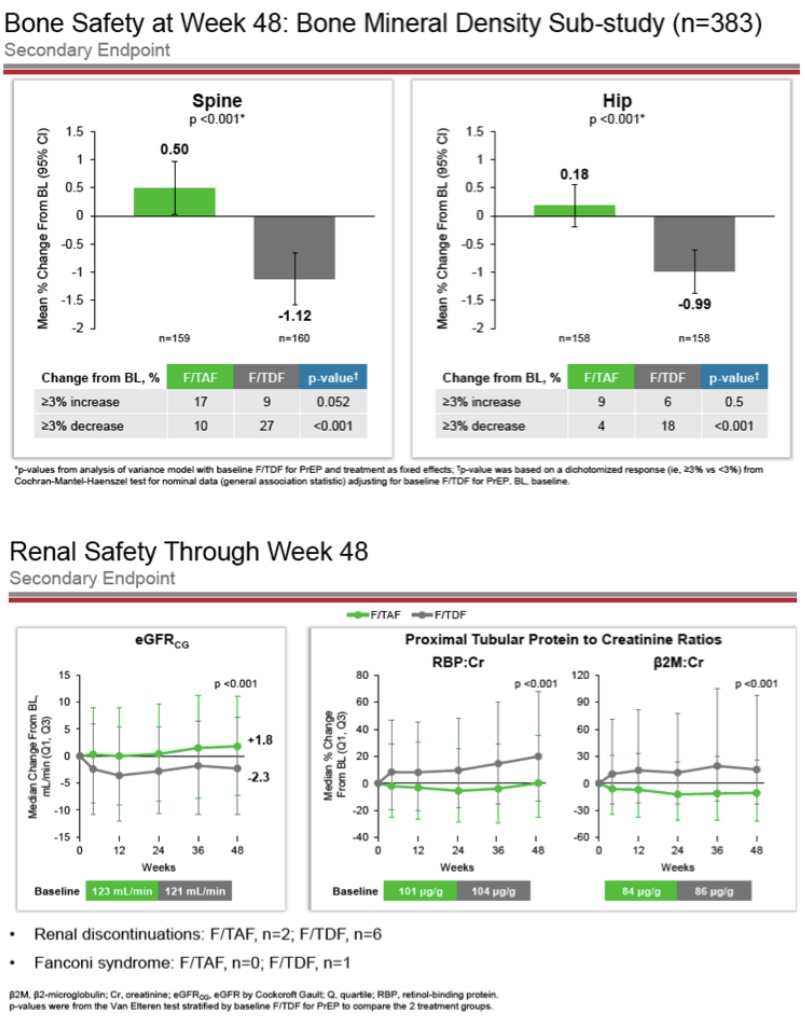
Among the trial’s roughly 5,400 matriculating participants (larger than any other PrEP trial to date), there were only 22 total seroconversions, 15 in the F-TDF arm and seven in the F-TAF arm (“53% reduction in HIV incidence for Descovy over Truvada” the company pointed out several times in its filing with the FDA). What’s more, the trial uncovered markedly improved bone- and renal safety outcomes in the F-TAF arm, all while delivering similar outcomes generally on side effects and adverse events. [Though it’s worth pointing out that Descovy was associated with weight gain and higher lipids/cholesterol in the trial, perhaps, perversely another reason why a sparing approach to dosing would be attractive to some consumers.] Such outcomes in safety and efficacy – especially given the pharmacological differences between the two forms of Tenofovir – underline not only how easily Descovy has found its way into the “superior” position in the minds of some consumers but also how easily they could make the logical leap from “superior product” to “effective even if untested.”
A ‘Superior’ Product?
“In the results presented at CROI there had been seven HIV infections in the Descovy arm and 15 in the Truvada arm. Since then, one more infection was seen in participants taking Descovy… this means that there were 46% fewer infections in the Descovy arm… [T]he 95% confidence interval of the study (its “fuzziness”) means that if one were to rerun the study 20 times, random effects would mean that the result could have turned out to be anything between 77% fewer infections on Descovy to 26% fewer on Truvada. So we can’t say Descovy has superior effectiveness as PrEP, though there is a trend in that direction [emphasis added].”
Gus Cairns in “96-week results of DISCOVER PrEP trial presented at European AIDS Conference,” 11/4/19, AIDSMap
The US NIAID Division of AIDS Director Carl Dieffenbach summarized those preliminary results with the “superiority” slip of tongue; “What we saw in the study was superior safety data from the use of TAF, but also a surprisingly small number of HIV infections as you’d expect because Truvada is pretty powerful as PrEP. TAF was superior … proven non-inferior to the standard. So, it’s looking good.”

In seizing on the fewer infections in the F-TAF arm of the trial, VOICE trial lead investigator Jeanne Marrazzo tweeted out from CROI 2019 “Eagerly awaited results of DISCOVERY [sic] trial demonstrates F/TAF nearly reached superiority over F/TDF as PrEP in MSM/TGW…”
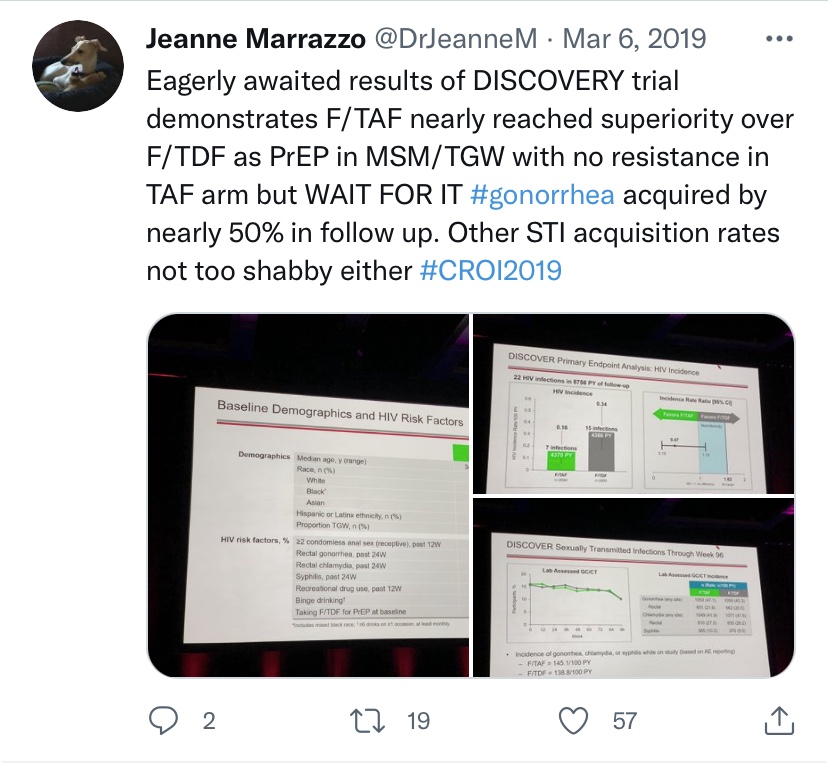
Gilead’s own Mike Elliot even got in on the Descovy-‘superiority’-same-sentence hyping tease action, telling the UK’s Gay Star News that week “It didn’t get to that superiority (over Truvada) but showed very good efficacy and numerically fewer transmissions than Truvada…And then there are those important measures on renal and bone…improved renal and bone safety.”
(The connotative effect of all this repeated positioning of the words ‘Descovy’ and ‘superior’ or ‘superiority’ [and ‘safe/safety’] so closely in communication, almost certainly, is not lost on any marketing professional worth their salt, regardless of the meaning of sentences uttered therein.)
THE LIMITATIONS (DATA/GUIDANCE GAPS)
No Good Data
Despite the encouraging inferential animal data, pharmacological data, trial data, and related fanfare, there are still data gaps as well as some pharmacological data that has given experts pause as to whether or not F-TDF could reach sufficiently protective levels in rectal tissues (or other local vulnerable tissues) when administered in an on-demand fashion like the 2-1-1 approach.
To repeat IPERGAY trial lead Jean-Michel Molina yet again, there just isn’t any clinical efficacy data on TAF-based 2-1-1 oral PrEP like there is with TDF-based 2-1-1 oral PrEP (IPERGAY, IPERGAY OLE, AmPrEP, the San Francisco study, PREVENIR, etc.). Indeed, it’s not at all clear:
- who would be doing that clinical research,
- with what demographic population, or even
- where F-TAF nondaily PrEP would be studied.
Nor are there any such completed/ongoing/pending HIV-related F-TAF-based intermittent prophylaxis clinical studies in the pipeline if you go by the recent listings in the US’ database of clinical trials. Indeed, it’s hard to overemphasize how big a limitation the lack of clinical efficacy data is for those intrepid few forging ahead on deploying F-TAF for 2-1-1.
Puzzling PK Data
And if that weren’t enough, the animal challenge trial data that informed the DISCOVER F-TAF PrEP trial had the curious finding of lower rectal mucosal drug levels than F-TDF PrEP. The finding was echoed in preliminary human pharmacological data among both HIV positive- and HIV negative human subjects. It raised the question anew of the systemic-vs-local protection Tenofovir-based PrEP provides via drug levels in rectal-, vaginal-, and penile tissues (usually the 1st contact points of HIV in the body) vs lymphatic cells/tissues and other related blood cells (the systems wherein HIV travels, lodges itself, hides, and replicates). The question and concern formed the basis of warnings from Robert Grant against premature deployment of F-TAF daily PrEP (warnings not unlike those of Molina later concerning 211 F-TAF PrEP) as Grant justified and embarked upon the (ultimately successful) DISCOVER trial.
No Guidance
Moreover, if those data gaps and inconsistencies weren’t enough, then further pause might be provided by the fact that no national- or international health authority has endorsed, recommended, or provided guidance for F-TAF 2-1-1 oral PrEP dosing: US Centers for Disease Control’s (CDC) 2021 PrEP recommendations
- Not the San Francisco AIDS Foundation’s (SFAF) San-Fran-did-intermittent-PrEP-1st-on-US-soil-and-coined-the-term-2-1-1-PrEP 2019 Pre Exposure Prophylaxis Health Program Magnet Clinical Protocol,
- Not the World Health Organization’s (WHO) 2-1-1-PrEP-specific 2019 Technical Update or even its hot-off-the-presses 2021 Updated HIV prevention, infant diagnosis, antiretroviral initiation and monitoring 2021 recommendations,
- Not the International Antiviral Society–USA’s (IAS-USA) 1st-in-America 2-1-1 PrEP-inclusive 2020 Recommendations for Antiretroviral Drugs for HIV Treatment and Infection Prevention,
- Not the European AIDS Clinical Society’s (EACS) HIV treatment/prevention 2020 guidelines,
- Not the vanguard-on-continent, South Africa’s 2020 recommendations.
- Not France’s latest glossy, popular AIDES 2021 PrEP 2-1-1 guide, and
- Not even the long-awaited US Centers for Disease Control’s (CDC) 2021 PrEP recommendations.
Thus, without convincing F-TAF 2-1-1 PrEP-specific preclinical- and clinical data and/or even said institutional guidance, some intrepid health care providers and consumers can only be described as getting ‘out over their skis’ with this innovation.
CONCLUSION
TL:DR
In the final analysis, we just can’t say for sure quite yet whether or not Descovy is effective with the 2-1-1-/event-based-/event-driven-/intermittent-/on-demand-/IPERGAY dosing protocol. This is because – even though there is some encouraging circumstantial evidence – we’ve seen no published large-scale clinical safety/efficacy data so far on oral F-TAF-PrEP 2-1-1 dosing. Hence, despite encouraging PK data suggesting F-TAF could allow for PrEP 211 dosing and or at least more forgiving daily dosing, the F-TAF daily oral PrEP FDA approval last year was limited, and such premature 2-1-1 F-TAF PrEP use otherwise has been prominently discouraged by the 2-1-1 expert.
Simple Solution: Study it?
The current COVID-19 pandemic underlines the need for rigorous scientific data upon which to base our public health decisions (though, ironically, it has also interrupted HIV treatment- and prevention research). Accordingly, the simple solution would be just to do a double-blind, randomized, head-to-head- or placebo-controlled clinical trial in order to answer the question of F-TAF 2-1-1 PrEP dosing efficacy.
However, given Gilead’s initial legal hesitance toward any TDF-based oral PrEP and the company’s articulated financial rationale against seeking approval for F-TDF 2-1-1 PrEP, it may never pursue study/approval of Descovy 211 PrEP. Indeed, it doesn’t take a stretch of the imagination to see that tighter dosing margins of error could be perceived by the company as opening it up once again to legal liability if seroconversion occurs “in the wild.” Similarly, the option of less TAF used per-consumer could be perceived as cutting into company profits.
Victim of PrEP’s Success?
Furthermore, another reason we may never get data to definitively answer this question is the complicated nature of completing PrEP trials in a clinical research landscape that includes safe-and-highly-effective TDF-based oral PrEP as part of the standard of care for trial participants. In other words, the more effective tools we have for preventing HIV, the more of these tools must be offered to future prevention trial participants to meet ethical standards. Also, the more effective these tools, the more future trial participants and/or time needed to meet statistical requirements for definitive trial outcomes.
Beyond that trial-design hurdle, getting funding from federal sources for this kind of research would be a long shot in a crowded pipeline under shifting research priorities. For instance, paradigm-shifting “Biomedical Prevention Era” developments including “PrEP 2.0” technologies LAI CAB, the DapiviRing, and AMP exiting the research/development pipeline recently, plus game-changing “PrEP 2.0” Phase III ISL (MK-8591) PrEP monthly oral pill trials along with the Phase II PrEPVacc and Phase III MOSAICO HIV vaccine trials having already crowded into that same pipeline. Such advancements and potential advancement as research funding competitors make it hard to justify such a relatively retrograde and niche technology as F-TAF 211 oral PrEP for cisMSM only. This is especially true in the face of NIH funding shifts away from including locally active PrEP technologies (gels, inserts, douches, suppositories, etc.) and toward only supporting systemic PrEP agents (pills, injections, implants, etc.).
A Glimmer of (Research) Hope?
Given this lacking information and funding, you might be as fascinated as I was to find a tantalizingly opaque assurance communicated by the San Francisco AIDS Foundation (SFAF) that there is some such TAF-based 2-1-1 oral PrEP research already underway…. At least if you believe SFAF’s recent rundown on TAF/FTC vs TDF/FTC oral PrEP.

(I had reached out to the SFAF to learn more about its posted “Descovy for PrEP 2-1-1 … being studied,” but, sadly, was given no such information. However, I’d encourage anyone else similarly-interested to reach out to SFAF as well about its “Resource: Side-by-side comparison: Truvada and Descovy for PrEP, San Francisco AIDS Foundation,” Medical review by Janessa Broussard, NP, November 2019, Prep@sfaf.org, https://www.sfaf.org/contact/https://www.sfaf.org/contact/.)
Final Words of Wisdom
In all honesty, the repeated posing of this question about TAF-based 2-1-1 oral PrEP should itself be taken as a market demand signal. In fact, actual use has even already been documented among trans men. As such, some may call this whole conversation one of irresponsible messaging. However, since we may never have the necessary data to answer the question definitively, we would do best to take a harm reductionist’s approach while integrating a shared decision-making model. That means being honest about what we do and don’t know in order to support patients own deliberations. That reminds me of the advice Bob Grant gave when discussing how to help patients navigate the tricky issue of sex planning and how long till protective drug levels; “ … In our effort to do no harm and be conservative, we may end up doing harm. And so, I think in the end, we’re better off just focusing on what we know, what we don’t know, and being very curious about what our patients are really asking [minute-mark 78;54].”
Now, this doesn’t mean that, in the case of F-TAF 2-1-1 oral PrEP, we follow in the footsteps just yet of maverick medical pros like Marcus Conant, Mike Youle, and Pietro Vernazzo when they prescribed TDF-PrEP off-label over a decade ago. However, it does mean we acknowledge that we know TAF produces higher drug levels than TDF in some places (but not others) and that it does also work as one component of a daily PrEP combo pill (for some populations). We don’t know if that translates to the same outcomes for 2-1-1 F-TAF oral PrEP, nor is that currently recommended.
Thus, it’s your-body-your-choice when it comes to how you protect yourself, but the ole caveat emptor is definitely in order that Descovy users who deploy it for 2-1-1 are doing so at their own (calculated) risk. So, it’s always a good idea to consult with your care provider and consider the relevant facts before you decide on the HIV prevention approach or approaches that work best for you.